Overview
DermTech is attempting to reduce the need for costly, invasive and inconvenient methods currently required to detect skin cancer. The company uses a patch, resembling a circular band-aid, which patients can place on themselves and then mail to DermTech’s laboratory where the subsequent testing is done on the collected sample. The laboratory uses molecular testing on skin cell samples, to check for two genes associated with melanoma.
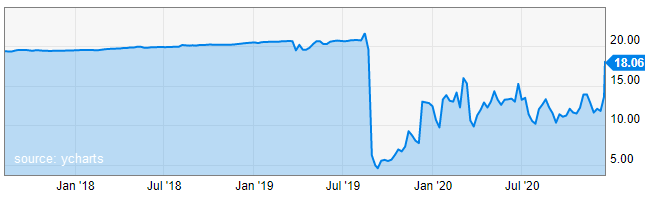
Why it Moved
On December 17th, 2020 the topline results of Dermtech’s study was announced. These results confirmed the high negative predictive value of (Pigmented Lesion Assay) PLA at 99% and found no significant adverse outcomes after long-term follow-up of PLA negative tests.
The stock price moved up 5.85% today, and 27.55% in afterhours on the positive results of the study.
Opportunity
Early detection can be life-saving for some cancer patients, and with the added convenience of in home detection, and potentially lower costs DermTech could continue to garner support from both the clinical and insurance side. In November, DermTeck announced it has entered an agreement with Blue Cross Blue Shield of Illinois.
While this is still an early stage pre-revenue company and carries a lot of risk - it also has a lot of potential upside, with a market cap around 300 million. And as company has just recently received approved and launching to market, there will likely be more news and continued catalysts for the stock to make waves in the coming months. We see a large market for DermTeck especially with the rapidly aging population. It is estimated that 1 in 5 adults will develop skin cancer by age 70. And even with younger adults, the use of tanning beds in recent decades has increased Melanoma rates, which is the deadliest form of skin cancer.
This site references only our opinion and is for information purposes only. It is not intended to be investment advice. Seek a duly licensed professional for investment advice. Disclaimer: We are long DMTK June 2020 calls
